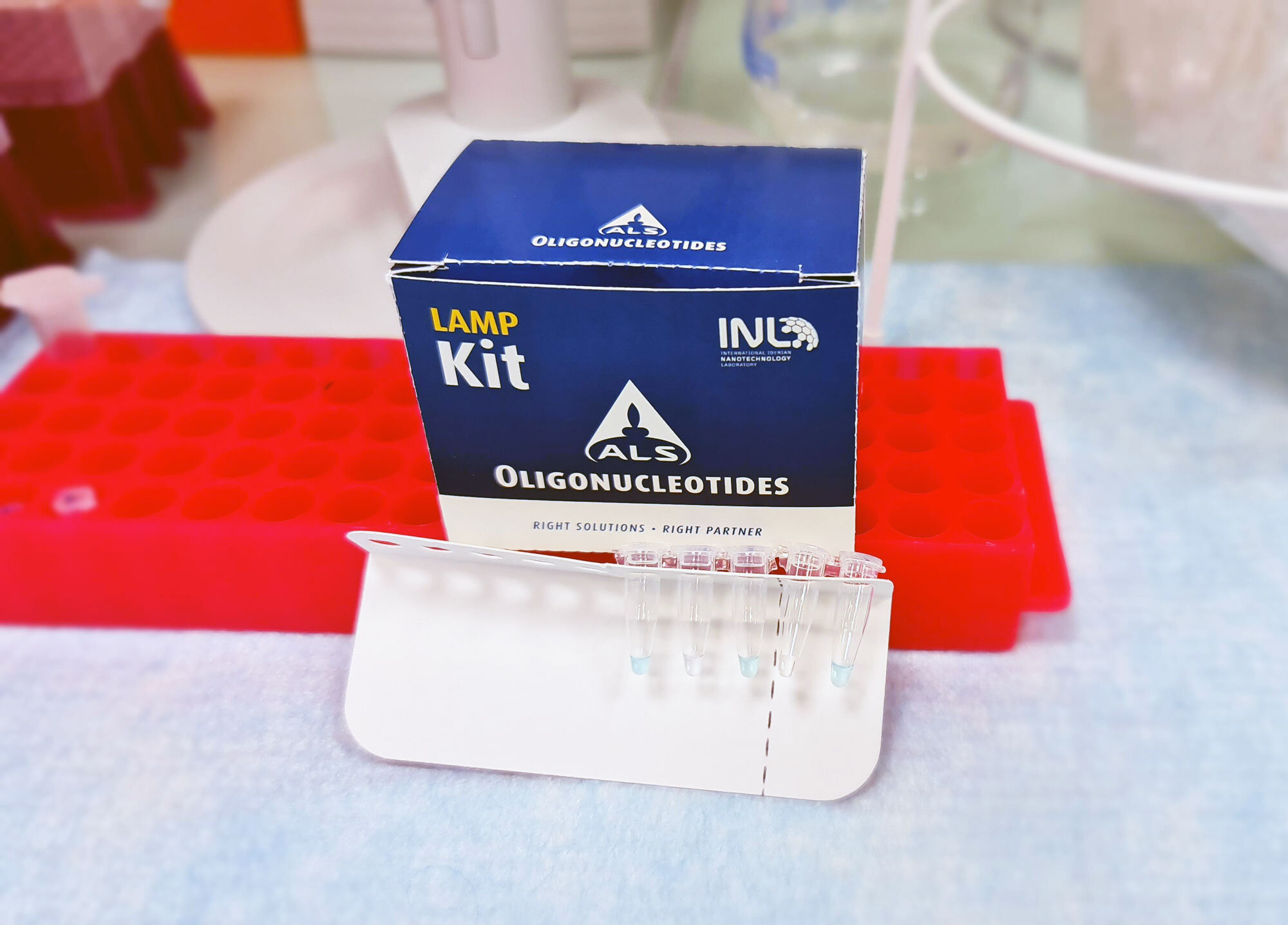
INL and ALS develop a COVID-19 test that is cheaper and as reliable as a PCR
June 11, 2021
INL – International Iberian Nanotechnology Laboratory, in partnership with ALS Life Sciences Portugal, announced the launch of an ‘in Vitro’ diagnostic kit for covid-19 that allows results to be obtained in around 45 minutes.
This SARSCoV-2 RT-LAMP kit, developed in a record time, has high levels of sensitivity (96%) and specificity (98%) and allows to carry out the tests in a fraction of the cost and time required by conventional PCR tests, ensuring similar quality in diagnosis.
The result is achieved in about 45 minutes, but this time can be reduced to 25 minutes if a fluorescence detection device is used in the laboratory.
The new kit, initially developed by INL staff researcher Alejandro Garrido-Maestu, uses RT-LAMP technology (rapid molecular nucleic acid amplification test) and “stands out for being faster than the real-time PCR alternative and simpler in the process implementation, dispensing the use of a complex laboratory apparatus, as it is carried out at constant temperature”.
In addition, the kit offers the possibility of visual interpretation of results by changing the colour, with a performance comparable to the reference PCR method, he adds.
Designed for the global market, the kit can have a huge impact in healthcare units, regions or countries where the available technology is low-grade and the teams of technicians or health professionals have lower levels of training.