NESC group’s recent advances in hydrogen research
July 21, 2020
Hydrogen has recently become “hot” and been frequently mentioned by politicians, researchers, policymakers and industrial stakeholders. As a clean and carbon-free energy carrier, hydrogen plays an essential role in the decarbonisation of various sectors, particularly the heavy industry and freight road transport where electrification is impossible or too costly.
Water electrolysis powered by renewable energy such as solar and wind is a promising and the only approach to producing “green” hydrogen. Early this year, the European Commission has launched a “2´40GW Green Hydrogen Initiative”, aiming to promote a massive increase of electrolysing production within the EU in order to support green hydrogen production. Many member states including Portugal and Spain have also drawn up the national strategy for hydrogen (e.g. EN-H2 in Portugal).
To produce hydrogen from water electrolysis, catalyst plays an important role and an efficient and durable catalyst may substantially lower the cost of electrolysed hydrogen, making it economically competitive to the hydrogen produced by conventional steam reforming. The Nanomaterials for Energy Storage and Conversion (NESC) group has been working on the development of water splitting electrocatalysts since 2014. The group has systematically investigated transition metal phosphide catalysts for use in both alkaline water electrolysis (AWE) and proton exchange membrane water electrolysis (PEMWE).
Continuing to work along this line, the group has recently made new advances in both AWE and PEMWE. The major challenge for AWE being employed in the industry is the low operation current density (usually below 0.5 A/cm2), which significantly limit the hydrogen yield in unit time. The NESC group reported a bifunctional porous cobalt phosphide foam electrode which can serve as both anode and cathode of an electrolyser, enabling AWE to occur at a high current density of 1 A/cm2 at around 2 V. Moreover, the group demonstrate extraordinary catalytic stability of 4000 hours. This renders the porous cobalt phosphide foam electrode a substantial promise for use in practical alkaline water electrolysis. A paper entitled “Bifunctional porous cobalt phosphide foam for high-current-density alkaline water electrolysis with 4000-h long stability” has been published in ACS Sustainable Chemistry & Engineering. Master student Yue Li is the first author of this paper. This work was done in collaboration with the Center of Chemistry, University of Minho.
Figure 1. Schematic illustration of the fabrication of porous cobalt phosphide foam electrode. (Bottom) Electrochemical performance of the alkaline electrolyzer composed of porous cobalt foam anode and cathode.
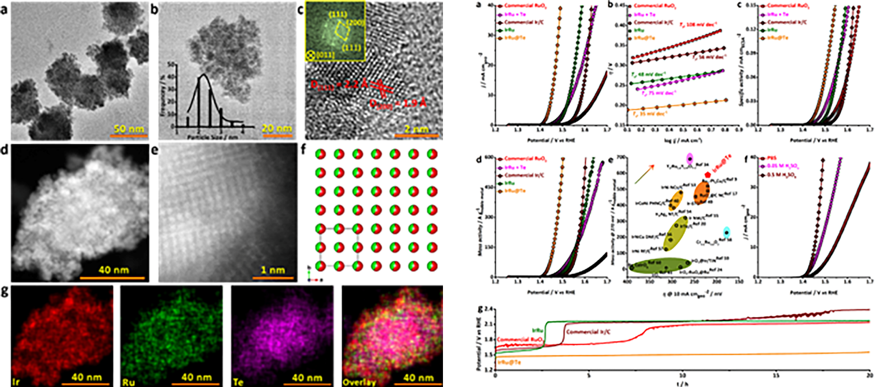
In addition, the NESC group recently also developed new catalysts showing outstanding catalytic performance for PEM electrolysis. Specifically, the group reported ultrafine iridium-ruthenium nanoclusters supported on conductive and acid-stable tellurium nanoparticles, which exhibited excellent activity and stability toward the oxygen evolution reaction – the bottleneck of water electrolysis for hydrogen production – in both strongly acidic and neutral solutions. A paper entitled “Strong electronic coupling between ultrafine iridium-ruthenium nanoclusters and conductive, acid-stable tellurium nanoparticle support for efficient and durable oxygen evolution in acidic and neutral media” has been published in ACS Catalysis and the paper has been viewed 1132 times since its publication online at the end of February. Research Fellow Junyuan Xu is the first author of this paper. This work is a result of collaboration with the Institute of Metal Research, Chinese Academy of Sciences and the Atomic Manipulation for Quantum Nanotechnology Group.
Figure 2. (Left) Microstructural characterization of the new IrRu@Te catalysts. (Right) Electrocatalytic performance of IrRu@Te catalysts.
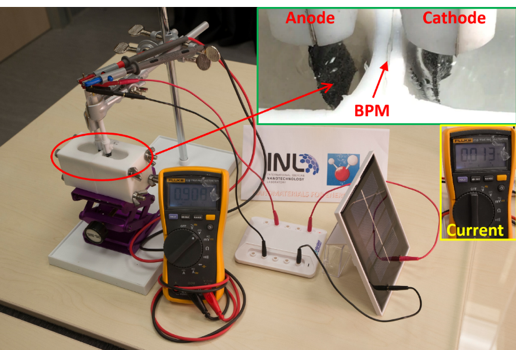
Furthermore, the NESC group is committed to developing bipolar membrane water electrolysis technology. The usage of the bipolar membrane allows the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) to take place simultaneously under kinetically favourable conditions, using exclusively low-cost, earth-abundant electrocatalysts.
Figure 3. Demonstration of solar-driven hydrogen production based on bipolar membrane asymmetric water electrolysis.
The group has demonstrated that HER and OER can be accomplished in strongly acidic and alkaline solutions, respectively, using bifunctional self-supported cobalt-nickel phosphide nanowire electrodes as anode and cathode and a bipolar membrane to separate them. Interestingly, the researchers found that if a “defective” bipolar membrane is used, the overall water electrolysis voltage needed can be significantly reduced such that the water electrolysis can be powered by a silicon solar panel at a low voltage of ca. 0.9 V. This work was published by invitation in Carbon Energy. Research Fellow Junyuan Xu and PhD student Isilda Amorim are co-first authors.