“Pushing myelination” a new article published in The Journal of Cell Science
August 14, 2020
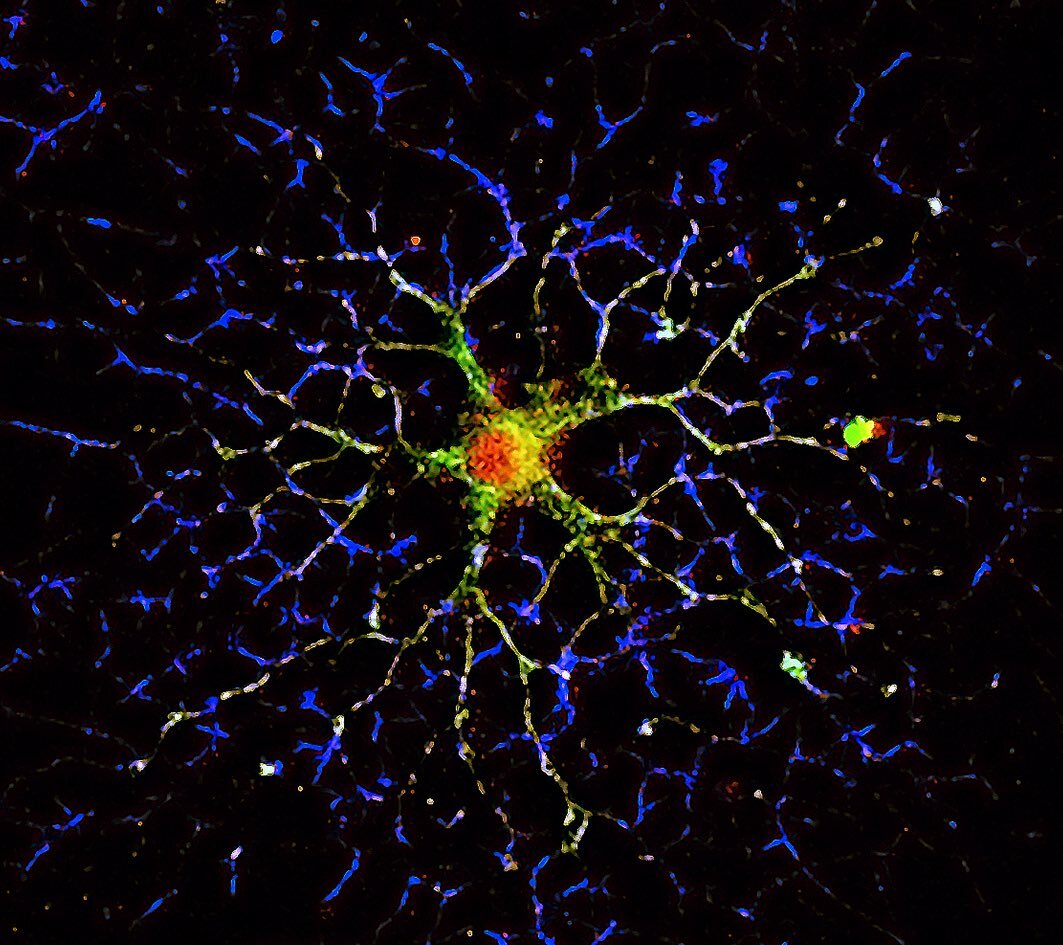
Gene expression and protein synthesis of non-muscle myosin NM2C and myosin Myo18a are upregulated during development of oligodendrocytes at a stage of active elaboration of myelin. In our study, both proteins are suggested to play a role in the production of the oligodendrocyte myelin membrane, the myelin sheet. The immunofluorescence image shows a mature oligodendrocyte under the developmental process of myelination. Myosin 18a is shown in red, α-tubulin in green and F-actin in blue.
Image taken by Amr Almaktari.
A new article by Helena Sofia Domingues, Mateusz M. Urbanski, Sandra Macedo-Ribeiro, Amr Almaktari, Azka Irfan, Yamely Hernandez, Haibo Wang, João Bettencourt Relvas, Boris Rubinstein, Carmen V. Melendez-Vasquez & Inês Mendes Pinto, was published in The Journal of Cell Science.
In this recent publication entitled “Pushing myelination – developmental regulation of myosin expression drives oligodendrocyte morphological differentiation”, researchers analyzed the expression pattern of myosin motors during oligodendrocyte development and reported that oligodendrocyte differentiation is regulated by the synchronized expression and non-uniform distribution of several members of the myosin network, particularly non-muscle myosins 2B and 2C, which potentially operate as nanomechanical modulators of cell tension and myelin membrane expansion at different cell stages.
Oligodendrocytes are highly specialized cells of the central nervous system whose main function is to produce myelin, a fatty membrane sheet that is used to wrap around axons to provide insulation and metabolic support of neuronal networks. In order to produce the myelin sheet, oligodendrocytes must undergo drastic alterations in their morphology through modulation of their cytoskeleton. In this collaborative and multidisciplinary work, they added a mechanical perspective to the process of oligodendrocyte myelination. They characterized a family of specific molecules called myosins that operate as nanomotors on the oligodendrocyte cytoskeleton to modulate its architecture and dynamics. Given their different mechanochemical properties, they found that these myosins are expressed and function in a time- and space-specific manner to coordinate the myelination process. In particular, they provided strong evidence that these molecules may operate as modulators of mechanical plasticity by regulating cell tension and myelin membrane expansion at different cell stages of differentiation.
+ You can access the article here.
+ You can also access Sofia Domingues interview here and here.